The history of the atom timeline project embarks on an extraordinary voyage, unveiling a captivating narrative brimming with groundbreaking discoveries and paradigm shifts. From the dawn of ancient Greek philosophy to the frontiers of modern physics, this project traces the evolution of our understanding of the fundamental building blocks of matter.
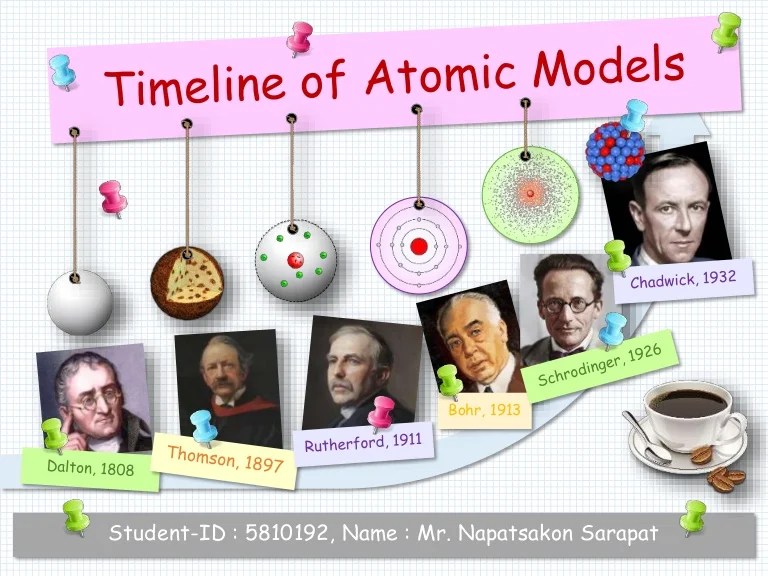
Throughout history, brilliant minds have dedicated their lives to unraveling the mysteries of the atom. From Democritus’s pioneering atomic theory to Dalton’s groundbreaking experiments, the quest for knowledge has propelled humanity forward.
Timeline of the History of the Atom: History Of The Atom Timeline Project

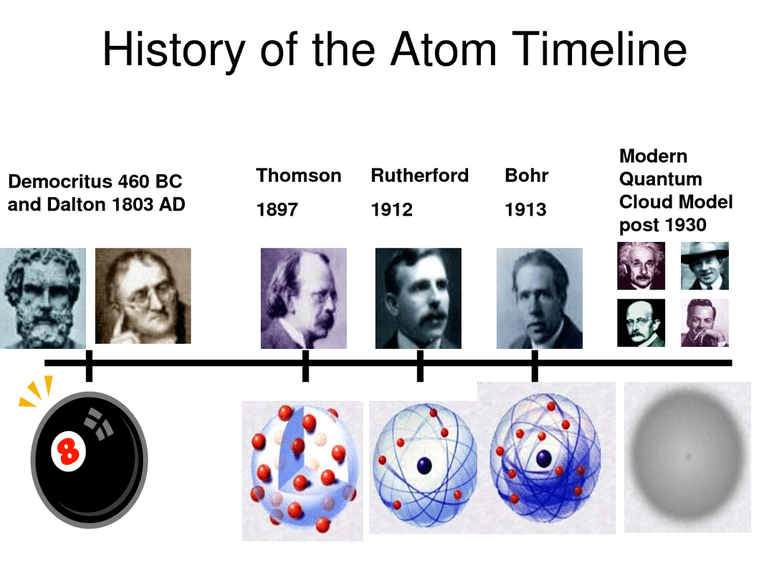
The history of the atom is a long and fascinating one, stretching back to the ancient Greek philosophers. Over the centuries, our understanding of the atom has evolved dramatically, thanks to the work of many brilliant scientists. Here is a timeline of some of the key events in the history of the atom:
Democritus and the Atomic Theory
In the 5th century BC, the Greek philosopher Democritus proposed that all matter is made up of tiny, indivisible particles called atoms. He believed that atoms were eternal, unchangeable, and could not be created or destroyed.
Dalton’s Atomic Theory, History of the atom timeline project
In the early 19th century, the English chemist John Dalton developed a more complete atomic theory. Dalton’s theory was based on the following postulates:
- All matter is made up of atoms.
- Atoms are indivisible and indestructible.
- All atoms of a given element are identical in mass and other properties.
- Atoms of different elements have different masses and properties.
- Atoms combine with each other in simple whole-number ratios to form compounds.
Thomson’s Discovery of the Electron
In 1897, the English physicist J.J. Thomson discovered the electron. Thomson’s experiment showed that electrons are much smaller than atoms and that they have a negative charge.
Rutherford’s Nuclear Model
In 1911, the New Zealand physicist Ernest Rutherford conducted a gold foil experiment that led to the development of the nuclear model of the atom. Rutherford’s experiment showed that most of the atom’s mass is concentrated in a tiny nucleus at the center of the atom.
The nucleus is surrounded by electrons, which orbit the nucleus in shells.
Bohr’s Atomic Model
In 1913, the Danish physicist Niels Bohr proposed a model of the atom that explained how electrons orbit the nucleus. Bohr’s model was based on the idea that electrons can only occupy certain orbits, and that the energy of each orbit is quantized.
Quantum Mechanics and the Modern Atomic Model
In the early 20th century, the development of quantum mechanics led to a new understanding of the atom. Quantum mechanics showed that electrons are not simply particles, but also have wave-like properties. The modern atomic model is based on quantum mechanics and takes into account the wave-particle duality of electrons.
Applications of Atomic Theory
The understanding of the atom has led to many technological advancements and applications in various fields, including:
- Nuclear energy
- Medicine
- Materials science
Answers to Common Questions
What was Democritus’s atomic theory?
Democritus proposed that all matter is composed of indivisible and indestructible particles called atoms, which vary in size and shape.
How did Dalton’s experiments contribute to the atomic theory?
Dalton’s experiments with gases led him to postulate that elements are composed of tiny, indivisible particles with specific masses.
What was the significance of Rutherford’s gold foil experiment?
Rutherford’s experiment demonstrated that most of the atom’s mass is concentrated in a tiny, positively charged nucleus.